| RESEARCH PAPER |
|
 |
|

|
|
|
| 1D Energetic Metal-organic Frameworks: Synthesis and Properties |
| Wei LIU,Houhe CHEN
|
| School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094 |
|
|
|
|
Abstract To prepare high-energy green initiating explosive, an one-dimension energetic metal-organic frameworks (1D EMOFs) , [Fe(ATZ)(H2O)4·2H2O]n, based on sodium 5,5'-azotetrazolate salt and ferrous ion has been synthesized by solvent evaporation method. The structure of product was fully characterized by IR spectroscopy, elemental analysis and single-crystal X-ray diffraction. The thermal decomposition performance of product was researched by differential scanning calorimetry. In addition, detonation performances of compounds was predicted by arbitrary theory of the Kamlet-Jacobs method which bases on the largest exothermic principle. Moreover, the impact sensitivity of 1D EMOFs was also tested. Results show that the detonation heat, detonation pressure and detonation velocity of [Fe(ATZ)(H2O)4·2H2O]n were 2 294.73 kJ/mol, 34.54 GPa and 8.83 km/s, respectively, and the impact sensitivity was 39.0 cm. With characteristics of typical primers, the 1D EMOFs could be used as a high-energy green initiating explosive.
|
|
Published: 25 January 2018
Online: 2018-01-25
|
|
|
|
|

|
|
Synthesis device of [Fe(ATZ)(H2O)4·2H2O]n 1—Water bath;2—Thermometer;3—Graduated cylindrical separatory funnel;4—Clip;5—Flask with two necks; 6—Iron support
|

| Compound | [Fe(ATZ)(H2O)4·2H2O]n | | Empirical formula | C2H12FeN10O6 | | Formula weight | 328.07 | | Crystal system | Triclinic | | Space group | P-1 | | T/K | 170 | | a/? | 6.209 1 | | b/? | 6.910 0 | | c/? | 7.852 1 | | α | 76.122 | | β | 74.308 | | γ | 70.427 | | V/?3 | 301.43 | | Z | 1 | | Density, calculated/(g/cm3) | 1.807 18 | | μ/mm-1 | 1.298 | | F(000) | 168 | | Independent reflections | 1 061 | | Data/restraint/parameters | 1 061/9/100 | | Goodness-of-fit on F2 | 1.135 |
|
|
Crystal data and structure refinement parameters for [Fe(ATZ)(H2O)4·2H2O]n
|
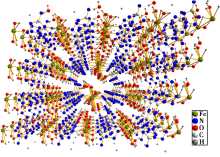
|
|
Crystal structure of [Fe(ATZ)(H2O)4·2H2O]n
|

|
|
Coordination mode of Fe2+ and ATZ2-
|

|
|
Infrared spectrum of [Fe(ATZ)(H2O)4·2H2O]n
|

|
|
DSC curve of [Fe(ATZ)(H2O)4·2H2O]n
|

| Compound | O2(g) | CO2(g) | H2O(g) | Fe2O3(s) | N2(g) | NH3(g) | C(s) | | Δf/(kJ·mol-1) | 0 | -393.51 | -241.82 | -824.25 | 0 | -46.11 | 0 |
|
|
Standard molar enthalpy of formation of several substances
|

| Compound | Δc
kJ·mol-1 | Q
kJ·mol-1 | N
mol·g-1 | M
g·mol-1 | ρ0
g·cm-3 | D
km·s-1 | P
GPa | IS
cm | | [Fe(ATZ)2(H2O)4]n | -3 424.36 | 2 294.73 | 0.03 | 22.40 | 1.81 | 8.83 | 34.54 | 39 |
|
|
Detonation parameters and sensitivity of [Fe(ATZ)(H2O)4·2H2O]n
|
| 1 | Zhang T L, Wu B D, Yang L . Recent research progresses in energetic coordination compounds[J]. Chinese Journal of Energetic Materials, 2013,21(2):137(in Chinese). | | 2 | 张同来, 武碧栋, 杨利 , 等. 含能配合物研究新进展[J]. 含能材料, 2013,21(2):137. | | 3 | Wang W, Chen S, Gao S . Syntheses and characterization of lead (Ⅱ) N, N-bis [1 (2) H-tetrazol-5-yl] amine compounds and effects on thermal decomposition of ammonium perchlorate[J]. European Journal of Inorganic Chemistry, 2009,2009(23):3475. | | 4 | Wu B D, Zhou Z N, Li F G , et al. Preparation, crystal structures, thermal decompositions and explosive properties of two new high-nitrogen azide ethylenediamine energetic compounds[J]. New Journal of Chemistry, 2013,37(3):646. | | 5 | Wu B D, Yang L, Wang S W , et al. Preparation, crystal structure, thermal decomposition, and explosive properties of a novel energetic compound [Zn (N2H4)2(N3)2]n: A new high-nitrogen material (N=65.60%)[J]. Zeitschrift für Anorganische und Allgemeine Chemie, 2011,637(3-4):450. | | 6 | Wu B D, Bi Y G, Li F G , et al. A novel stable high-nitrogen energetic compound: Copper (Ⅱ) 1, 2-diaminopropane azide[J]. Zeitschrift für Anorganische und Allgemeine Chemie, 2014,640(1):224. | | 7 | Zhang Q, Shreeve J M . Metal-organic frameworks as high explosives: A new concept for energetic materials[J]. Angewandte Chemie International Edition, 2014,53(10):2540. | | 8 | Zhang S, Yang Q, Liu X , et al. High-energy metal-organic frameworks (HE-MOFs): Synjournal, structure and energetic performance[J]. Coordination Chemistry Reviews, 2016,307:292. | | 9 | Qin J S, Zhang J C, Zhang M , et al. A highly energetic N-rich zeolite-like metal-organic framework with excellent air stability and insensitivity[J]. Advanced Science, 2015,2(12):1500150. | | 10 | Bushuyev O S, Brown P, Maiti A , et al. Ionic polymers as a new structural motif for high-energy-density materials[J]. Journal of the American Chemical Society, 2012,134(3):1422. | | 11 | Li S, Wang Y, Qi C , et al. 3D energetic metal-organic frameworks: Synjournal and properties of high energy materials[J]. Angewandte Chemie International Edition, 2013,52(52):14031. | | 12 | Qu X, Zhai L, Wang B , et al. Copper-based energetic MOFs with 3-nitro-1 H-1, 2, 4-triazole: Solvent-dependent syntheses, structures and energetic performances[J]. Dalton Transactions, 2016,45(43):17304. | | 13 | Liu X, Gao W, Sun P , et al. Environmentally friendly high-energy MOFs: Crystal structures, thermostability, insensitivity and remarkable detonation performances[J]. Green Chemistry, 2015,17(2):831. | | 14 | Qu X N, Zhang S, Wang B Z , et al. An Ag (Ⅰ) energetic metal-organic framework assembled with the energetic combination of furazan and tetrazole: Synjournal, structure and energetic performance[J]. Dalton Transactions, 2016,45(16):6968. | | 15 | Feng Y, Liu X, Duan L , et al. In situ synthesized 3D heterometallic metal-organic framework (MOF) as a high-energy-density material shows high heat of detonation, good thermostability and insensitivity[J]. Dalton Transactions, 2015,44(5):2333. | | 16 | Qu X, Zhang S, Yang Q , et al. Silver (Ⅰ)-based energetic coordination polymers: Synjournal, structure and energy performance[J]. New Journal of Chemistry, 2015,39(10):7849. | | 17 | Yang Q, Song X, Ge J , et al. A 2D nickel-based energetic MOFs incorporating 3, 5-diamino-1, 2, 4-triazole and malonic acid: Synjournal, crystal structure and thermochemical study[J]. The Journal of Chemical Thermodynamics, 2016,92:132. | | 18 | Fischer D, Klap?tke T M, Stierstorfer J . Potassium 1,1'-dinitramino-5,5'-bistetrazolate: A primary explosive with fast detonation and high initiation power[J]. Angewandte Chemie International Edition, 2014,53(31):8172. | | 19 | Tang Y, He C, Mitchell L A , et al. Potassium 4,4'-bis (dinitromethyl)-3,3'-azofurazanate: A highly energetic 3D metal-organic framework as a promising primary explosive[J]. Angewandte Chemie, 2016,128(18):5655. | | 20 | Sun Y, Yan D, Zhu S , et al. Synjournal and characterization of zinc 5, 5'-azotetrazolate[J]. Chinese Journal of Energetic Materials, 2012,20(3):297(in Chinese). | | 21 | 孙艳苓, 颜冬林, 朱顺官 , 等. 5, 5'-偶氮四唑锌的合成及表征[J]. 含能材料, 2012,20(3):297. | | 22 | Kamlet M J, Jacobs S . Chemistry of detonations. Ⅰ. A Simple method for calculating detonation properties of C-H-N-O explosives[J]. The Journal of Chemical Physics, 1968,48:23. | | 23 | Kamlet M J, Ablard J . Chemistry of detonations. Ⅱ. Buffered Equilibria[J]. The Journal of Chemical Physics, 1968,48:36. | | 24 | Kamlet M J, Dickinson C . Chemistry of detonations. Ⅲ. Evaluation of the simplified calculational method for Chapman-Jouguet detonation pressures on the basis of available experimental information[J]. The Journal of Chemical Physics, 1968,48:43. | | 25 | Kamlet M J, Hurwitz H . Chemistry of detonations. Ⅳ. Evaluation of a simple predictional method for detonation velocities of C-H-N-O explosives[J]. The Journal of Chemical Physics, 1968,48:3685. | | 26 | 4 Wang Y, Zhang J, Su H , et al. A simple method for the prediction of the detonation performances of metal-containing explosives[J]. The Journal of Physical Chemistry A, 2014,118(25):4575. | | 27 | 25 傅献彩, 沈文霞 , 等. 物理化学(上)[M]. 北京: 高等教育出版社. 2005. | | 28 | 26 GJB5891.22-2006. 机械撞击感度实验[S]. | | 29 | 27 蒋荣光, 刘自铴 . 起爆药[M]. 北京: 兵器工业出版社. 2006. |
|
|
|
|

 渝公网安备50019002502923号 © Editorial Office of Materials Reports.
渝公网安备50019002502923号 © Editorial Office of Materials Reports.
 2018, Vol. 32
2018, Vol. 32 