| RESEARCH PAPER |
|
 |
|

|
|
|
| Study of Corrosion Behavior of HSn62-1 in Acid,Alkali and Salt Solution |
| Xinlin LI1,Guo JIN1,Lixia KANG2,Xuejia PANG3,Weiqi CAO1,Haidou WANG4,Binshi XU4,Xiufang CUI1
|
1 Institute of Corrosion Science and Surface Technology, College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin 150001
2 Army Aviation Equipment Development Office, Beijing 100000
3 The 703 Research Institute of CSIC, Harbin 150078
4 Academy of Armored Force Engineering, National Key Laboratory for Remanufacturing, Beijing 100072 |
|
|
|
|
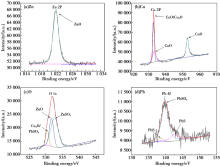
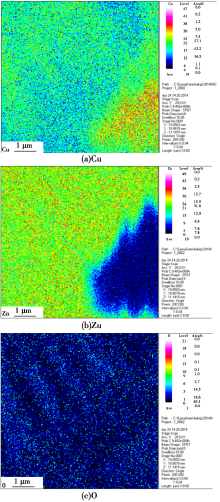
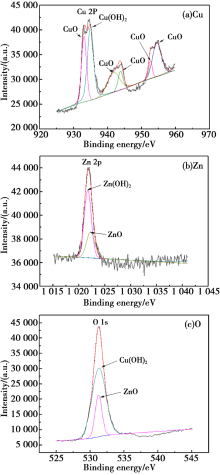
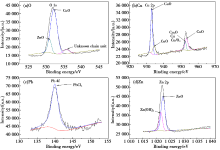
Abstract Scanning electronic microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), electro probe micro-analysis (EPMA), open-circuit potential and potentiodynamic polarization and X-ray photoelectron spectroscopy (XPS) were used to explore the corrosion mechanism and morphology of HSn62-1 brass in H2SO4 solution(pH=2),NaOH solution(pH=13) and NaCl solution(3.5%, mass fraction). The results indicated that there existed obvious weight loss in H2SO4 solution compare to NaOH solution and NaCl solution. Numerous corrosion products were detected except CuO and ZnO. The dezincification of HSn62-1 in H2SO4 solution can be explained as selective dissolution and its cathodic reactions were dominated by oxygen reduction and hydrogen reaction. In contrast, the dezincification mechanism was simultaneous dissolution of both zinc and copper and with the subsequent re-deposition of copper. Besides the cathodic reaction was hydrogen evolution corrosion in NaCl solution and oxygen evolution corrosion in NaOH solution.
|
|
Published: 25 January 2018
Online: 2018-01-25
|
|
|
|
|

|
|
Corrosion morphology of HSn62-1 immersed in acidic medium for:(a) 6 h, (b) 30 h
|
|
|
XPS analysis of corrosion products of HSn62-1 in H2SO4 solution (pH=2)
|
|
|
EPMA images of HSn62-1 immersed in H2SO4 (pH=2) for 30 h
|

|
|
Corrosion morphology of HSn62-1 immersed in NaOH solution for:(a) 6 h, (b) 30 h
|
|
|
XPS analysis for the corrosion products of HSn62-1 in NaOH solution (pH=13)
|

|
|
Surface scanning of HSn62-1 immersed in NaOH solution for 30 h
|

|
|
Corrosion morphology of HSn62-1 immersed in 3.5wt% NaCl for:(a) 6 h, (b) 30 h
|
|
|
XPS analysis of corrosion products of HSn62-1 in NaCl solution
|

|
|
EPMA images of HSn62-1 immersed in NaCl solution for 30 h
|

|
|
Mass loss curves of HSn62-1 with time in the three solutions
|
|
|
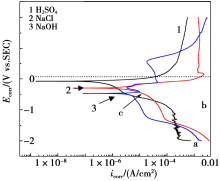
Potentiodynamic polarization curves of HSn62-1 in the three solutions
|
| 1 | Kumar S, Narayanan T S N, Manimaran A , et al. Effect of lead content on the dezincification behaviour of leaded brass in neutral and acidified 3.5% NaCl solution[J]. Materials Chemistry and Physics, 2007,106(1):134. | | 2 | Chen Jie, Zheng Qifei, Sun Shuangqin , et al. Long-term atmospheric corrosion behavior of naval brass HSn62-1[J]. The Chinese Journal of Nonferrous Metals, 2011,21(3):577(in Chinese). | | 3 | 陈杰, 郑弃非, 孙霜青 , 等. 海军黄铜HSn62-1的长期大气腐蚀行为[J]. 中国有色金属学报, 2011,21(3):577. | | 4 | 3 王超, 钟庆东, 周国治 , 等. H62黄铜在电解质溶液中的脱锌腐蚀机制研究[ C]∥2008年全国冶金物理化学学术会议专辑(上册).贵阳, 2008. | | 5 | El-Sherif R M, Ismail K M, Badawy W A . Effect of Zn and Pb as alloying elements on the electrochemical behavior of brass in NaCl solutions[J]. Electrochimica Acta, 2004,49(28):5139. | | 6 | 5 Radovanovi c ' M B , Petrovi c ' M B , Simonovi c ' A T , et al. Cysteine as a green corrosion inhibitor for Cu37Zn brass in neutral and weakly alkaline sulphate solutions[J]. Environmental Science and Pollution Research, 2013,20(7):4370. | | 7 | 6 Liu Jianfeng, Chen Yisheng, Zhu Zhiyun, et al. The effect of processing techniques on corrosion resistance of RE HSn62-1 brass[J].Aluminium Processing, 2009(2):24(in Chinese). | | 8 | 刘坚锋, 陈一胜, 朱志云 , 等. 加工工艺对稀土HSn62-1黄铜耐脱锌腐蚀性能的影响[J].铝加工, 2009(2):24. | | 9 | Zhao Yuehong, Lin Leyun, Wang Zhenghai . Influence of microstructure on local corrosion sensitivity of HSn70-1A copper alloy[J]. Corrosion Science and Protection Technology, 2011,23(6):479(in Chinese). | | 10 | 赵月红, 林乐耘, 王振海 . 显微组织对黄铜局部腐蚀敏感性的影响[J]. 腐蚀科学与防护技术, 2011,23(6):479. | | 11 | Zhang Juan, Tang Ning, Shang Yongjia , et al. The influence of alloy elements on the corrosion resistance of brass and mechanism[J]. Corrosion and Protection, 2012,33(7):605(in Chinese). | | 12 | 张娟, 唐宁, 尚用甲 , 等. 合金元素对黄铜耐腐蚀性能的影响和作用机理[J]. 腐蚀与防护, 2012,33(7):605. | | 13 | Yohai L, Vázquez M, Valcarce M B . Brass corrosion in tap water distribution systems inhibited by phosphate ions[J]. Corrosion Science, 2011,53(3):1130. | | 14 | Allam N K, Nazeer A A, Ashour E A . A review of the effects of benzotriazole on the corrosion of copper and copper alloys in clean and polluted environments[J]. Journal of Applied Electrochemistry, 2009,39(7):961. | | 15 | Valcarce M B, De Sanchez S R, Vazquez M . Brass dezincification in a tap water bacterial suspension[J]. Electrochimica Acta, 2006,51(18):3736. | | 16 | 12 路俊攀, 李湘海 , 中国有色金属工业协会. 加工铜及铜合金金相图谱[M]. 长沙: 中南大学出版社, 2010. | | 17 | Baruj A, Granada M, Arneodo Larochette P , et al. Primordial hexagonal phase formation during the bcc dezincification of the β Cu-Zn single crystalline surface: Matrix instabilization and transformation path[J]. Journal of Alloys and Compounds, 2009,481:129. | | 18 | Troiani H E, Ahlers M. The formation of an intermediate structure during the dezincification of β Cu-Zn alloys and its relevance for the martensitic transformation[J].Materials Science and Engineering: A 1999, 273- 275:200. | | 19 | Troiani H E, Baruj A. In situ optical microscopy study of a phase transformation induced by the dezincification of beta Cu-Zn[J]. Materials Science and Engineering: A 2007, s454- 455(16):441. | | 20 | Assouli B, Srhiri A, Idrissi H . Characterization and control of selective corrosion of α, β'-brass by acoustic emission[J]. NDT & E International, 2003,36(2):117. | | 21 | Wang Dan, Xie Fei, Wu Ming et al. Effect of cathode potentials on stress corrosion behavior of X80 pipeline steel in simulated alkaline soil solution[J]. Journal of Central South University (Science and Technology), 2014,45(9):2986(in Chinese). | | 22 | 王丹, 谢飞, 吴明 , 等. 阴极电位对X80管线钢在碱性土壤模拟溶液中应力腐蚀行为的影响[J]. 中南大学学报(自然科学版), 2014,45(9):2986. | | 23 | He H, Zhang T, Zhao C , et al. Effect of alternating voltage passivation on the corrosion resistance of duplex stainless steel[J]. Journal of Applied Electrochemistry, 2009,39(5):737. | | 24 | Sun Zhaodong, Du Min, Zhang Jin , et al. The cathodic polarization behavior of 316L stainless steel in seawater[J]. Materials Science & Technology, 2011,19(1):36(in Chinese). | | 25 | 孙兆栋, 杜敏, 张静 , 等. 316L不锈钢在海水中的阴极极化行为研究[J]. 材料科学与工艺, 2011,19(1):36. | | 26 | 20 魏宝明. 金属腐蚀理论及应用[M]. 北京: 化学工业出版社, 1984. |
|
|
|
|

 渝公网安备50019002502923号 © Editorial Office of Materials Reports.
渝公网安备50019002502923号 © Editorial Office of Materials Reports.
 2018, Vol. 32
2018, Vol. 32 