| MATERIALS AND SUSTAINABLE DEVELOPMENT: ADVANCED MATERIALS FOR CLEAN ENERGY UTILIZATION |
|
 |
|

|
|
|
| Preparation and Electrochemical Performance of Humic Acid-based Graphitized Materials |
| Dongyong SI,Guangxu HUANG,Chuanxiang ZHANG,Baolin XING,Zehua CHEN,Liwei CHEN,Haoran ZHANG
|
| College of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo 454003 |
|
|
|
|
Abstract The lithium-ion battery anode material have been prepared from humic acid through high-temperature heat treatment. The morphology, microcrystalline structure and electrochemical properties of as-prepared activated material were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) and electrochemical testing system. The results indicated that the humic acid-based graphitized materials showed a more regular graphite lamellar structure, and the degree of graphitization of the materials was also getting higher and higher with the increase of graphitization temperature. The humic acid-based graphitized mate-rials all presented good electrochemical performance. The graphitized material with the temperature of 2 800 ℃ had a first discharge specific capacity of 356.7 mAh/g and a charge capacity of 277.6 mAh/g, and the initial coulombic efficiencies was 77.81%. The capacity retention rate after 50 cycles at 1C and 2C rates was as high as 99.4% and 95.9%, respectively. The above results suggest that the humic acid-based graphitized material is an ideal lithium ion battery anode material.
|
|
Published: 10 February 2018
Online: 2018-02-10
|
|
|
|
|
|
|
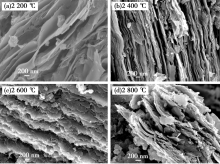
SEM patterns of Shanxi humic acid graphitized materials(2 000×)
|
|
|
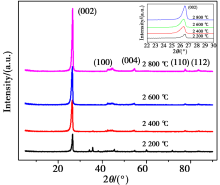
XRD patterns of Shanxi humic acid graphitized materials
|
|
|
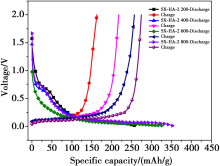
The charge/discharge curves of the Shanxi humic acid graphitized electrode materials in the first cycles with the current of 0.1C rate
|

| Sample | 0.5C
mAh/g | 1C
mAh/g | 2C
mAh/g | Capacity
retention rate | | SX-HA-2200 | 129.1 | 90.6 | 30.4 | 83.0% | | SX-HA-2400 | 126.6 | 105.6 | 38.5 | 89.3% | | SX-HA-2600 | 216.7 | 138.6 | 37.0 | 90.7% | | SX-HA-2800 | 239.4 | 200.6 | 150.7 | 94.2% |
|
|
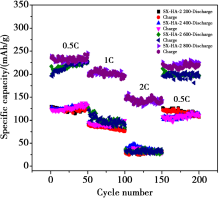
Discharge specific capacity and capacity retention at different current rates of Shanxi humic acid graphitizing electrode materials
|
|
|
The cycle performance and rate of the Shanxi humic acid graphitized electrode materials
|
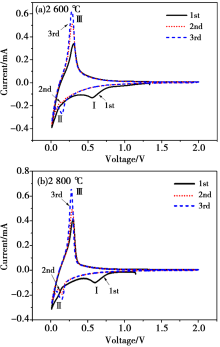
|
|
The cyclic voltammetry curve of Shanxi humic acid graphitized electrode material
|
| 1 | Tarscon J M, Rrmand M . Review article issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001,414:359. | | 2 | Armand M, Tarascon J M . Buliding better batteries[J]. Nature, 2008,451(7179):652. | | 3 | Xiao Q, Fan Y, Wang X , et al. A multilayer Si/CNT coaxial nanofiber LIB anode witha high areal capacity[J]. Energy & Environmental Science, 2014,2(7):655. | | 4 | Lv Y C, Li H . Review of basic problems about electrochemical energy storage[J]. Electro-chemistry, 2015,21(5):412(in Chinese). | | 4 | 吕迎春, 李泓 . 电化学储能基本问题综述[J]. 电化学, 2015,21(5):412. | | 5 | Sun X L . Preparation and electrochemical performance of carbon anode materials for lithiu-mion battery[D]. Qinhuangdao:Yanshan University, 2010(in Chinese). | | 5 | 孙学亮 . 锂离子电池碳负极材料的制备及其电化学性能的研究[D]. 秦皇岛:燕山大学, 2010. | | 6 | Zhang Y G . Modification and surface treatment of carbon materials used as anode lithium ion secondary battery[D]. Tianjin:Tianjin University, 2004(in Chinese). | | 6 | 张永刚 . 锂离子二次电池炭负极材料的改性与修饰[D]. 天津:天津大学, 2004. | | 7 | Wang W Y . Study on needle coke coated by phenolic resin used for lithium ion batteries[D]. Tianjin:Tianjin University, 2007(in Chinese). | | 7 | 王文燕 . 酚醛树脂包覆针状焦作为锂离子电池负极材料的研究[D]. 天津:天津大学, 2007. | | 8 | Liu S H , et al. Improving the electrochemical properties of natural graphite spheres by coating with a pyrolytic carbon shell[J]. New Carbon Materials, 2008,23(1):30. | | 9 | Zhang F L, Zhang S Y, Wu S . Present situation and future prospect of China graphite industry[J]. Carbon Technology, 2015,34(5):1(in Chinese). | | 9 | 张福良, 张世洋, 吴珊 . 中国石墨产业发展现状及未来展望[J]. 炭素技术, 2015,34(5):1. | | 10 | Shen W C , et al. Current situation and development of Chinese graphite industry[J]. China Nonferrous Metals Industry,2013(2):1(in Chinese). | | 10 | 沈万慈 , 等. 石墨产业的现状与发展[J].中国非金属矿工业导刊,2013(2):1. | | 11 | Zhao P , et al. Biotechnology humic acids-based electrospun carbon nanofibers as cost efficient electrodes for lithium-ion batteries[J]. Electrochimica Acta, 2016,203:66. | | 12 | Cheng L , et al. Research progress of humic-acid containing fertilizer[J].Soil and Fertilizer Sciences,2011(5):1(in Chinese). | | 12 | 程亮 , 等. 腐殖酸肥料的研究进展[J].中国土壤与肥料,2011(5):1. | | 13 | Han G H , et al. High-temperature oxidation behavior of vanadium, titanium-bearing magnetite pellet[J]. Journal of Iron and Steel Research, 2011,18(8):14. | | 14 | Qazi U Y, Javaid R . Composite nanostructures with metal components[J]. Advances in Nanoparticles, 2016,5(1):27. | | 15 | Kim B H , et al. Solvent-induced porosity control of carbon nanofiber webs for supercapacitor[J]. Journal of Power Sources, 2011,196(23):10496. | | 16 | Jia K , et al. Solution blown aligned carbon nanofiber yarn as supercapacitor electrode[J]. Journal of Materials Science:Materials in Electronics, 2013,24(12):4769. | | 17 | Zhao Y . Research of graphitized needle coke coated by phenolic resin pyrolytic carbon as anode material for lithium ion battery[D]. Shanghai:East China University of Science and Technology, 2012(in Chinese). | | 17 | 赵跃 . 酚醛树脂热解炭包覆石墨化针状焦用于锂离子电池负极材料的研究[D]. 上海:华东理工大学, 2012. | | 18 | Tian L L , et al. Insertion and release mechanism of lithium ion in graphene materials[J].Science China Press,2011(18):1431(in Chinese). | | 18 | 田雷雷 , 等.锂离子在石墨烯材料中的嵌入脱出机制[J].科学通报, 2011(18):1431. | | 19 | Lu M, Cheng H, Yang Y . A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells[J]. Electrochimica Acta, 2008,53(9):3539. | | 20 | Guo D C , et al. Preparation and electrochemical performance of expanded graphites as anode materials for a lithium-ion battery[J]. New Carbon Materials, 2015,30(5):419(in Chinese). | | 20 | 郭德超 , 等. 微膨石墨锂离子电池负极材料的制备及电化学性能[J]. 新型炭材料, 2015,30(5):419. |
|
|
|
|

 渝公网安备50019002502923号 © Editorial Office of Materials Reports.
渝公网安备50019002502923号 © Editorial Office of Materials Reports.
 2018, Vol. 32
2018, Vol. 32 