| MATERIALS AND SUSTAINABLE DEVELOPMENT: ADVANCED MATERIALS FOR CLEAN ENERGY UTILIZATION |
|
 |
|

|
|
|
| Effect of Anionic Groups of Cobalt Salt on the Electrocatalytic Activity of Co-N-C Catalysts |
| Yimeng XIA1,Shuai WU1,Feng TAN1,Wei LI1,Qingmao WEI1,Chungang MIN2,Xikun YANG1,2
|
1 College of Material Science and Engineering, Kunming University of Science and Technology, Kunming 650093
2 Research Center for Analysis and Measurement, Kunming University of Science and Technology, Kunming 650093 |
|
|
|
|
Abstract During the process of the aniline polymerization, bivalent cobalt salt with different anionic groups such as (C2H3O2)22-, Cl22-, (NO3)22-, SO42- and C2O42- were added into the solution and then different polyaniline cobalt (PANI-Co) coordination polymer were obtained. Finally, Co-N-C catalysts were prepared through pyrolysis of PANI-Co coordination polymer. The morphology, structure, chemical composition and chemical valence of the Co-N-C catalysts were characterized by scanning electron microscopy (SEM), X-ray spectroscopy (XRD), X-ray photoelectron spectroscopy (XPS) and Raman spectra (Raman). The electrocatalytic activity of Co-N-C catalysts were tested by electrochemical method. The results showed that the cobalt salt anionic groups had little impact on the morphology of Co-N-C catalysts, but had a great influence on the composition and surface chemistry of Co-N-C catalysts, carbon structure, degree of graphitization and the valence of Co. The cobalt salt anionic groups could affect the electrocatalytic activity of Co-N-C catalysts. The catalytic activities decreased as (C2H3O2)22->Cl22->(NO3)22->SO42->C2O42-. The Co-N-C catalysts prepared by cobalt salt containing (C2H3O2)22- and Cl22- anions had higher ORR activity, which possibly due to the higher content of graphite nitrogen and pyridine nitrogen.
|
|
Published: 10 February 2018
Online: 2018-02-10
|
|
|
|
|
|
|
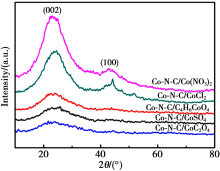
XRD patterns of different Co-N-C catalysts
|
|
|
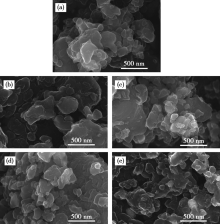
The SEM images of different Co-N-C catalysts:(a)Co-N-C/C4H6CoO4,(b)Co-N-C/CoCl2,(c)Co-N-C/CoSO4,(d)Co-N-C/Co(NO3)2,(e)Co-N-C/CoC2O4
|

| Sample | C 1s/% | N 1s/% | O 1s/% | S 2p/% | Co 2p/% | Co 2p/eV | | Co-N-C/C4H6CoO4 | 81.41 | 1.85 | 16.08 | 0.27 | 0.39 | 779.12 | | Co-N-C/CoCl2 | 81.72 | 2.39 | 15.13 | 0.15 | 0.61 | 779.25 | | Co-N-C/Co(NO3)2 | 83.21 | 2.34 | 13.98 | 0.08 | 0.39 | 779.65 | | Co-N-C/CoSO4 | 82.49 | 1.84 | 15.23 | 0.13 | 0.32 | 779.60 | | Co-N-C/CoC2O4 | 56.95 | 1.84 | 29.36 | 2.97 | 8.87 | 778.69 |
|
|
XPS results of the surface element analysis of Co-N-C
|

|
|
The XPS spectra of N 1s region for different Co-N-C catalysts
|

|
|
The XPS spectra of Co 2p region for different Co-N-C catalysts
|
|
|
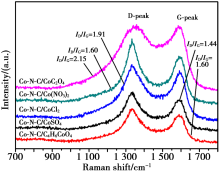
Raman spectra of different Co-N-C catalysts
|
|
|
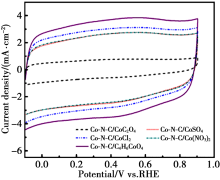
CV curves of different Co-N-C catalysts
|
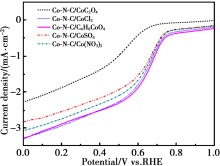
|
|
LSV curves of different Co-N-C catalysts
|
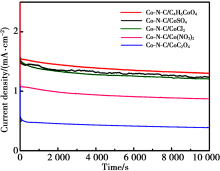
|
|
The different Co-N-C catalysts in O2-saturated 0.5 mol/L H2SO4 solution at 1 500 r/min
|

| Sample | n | | Co-N-C/C4H6CoO4 | 3.54 | | Co-N-C/CoCl2 | 3.85 | | Co-N-C/Co(NO3)2 | 3.90 | | Co-N-C/CoSO4 | 3.63 | | Co-N-C/CoC2O4 | 2.21 |
|
|
The n value of ORR of different Co-N-C catalysts at 0.25 V electrode potential
|
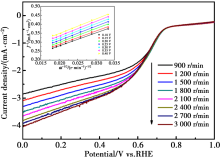
|
|
LSV curves at different rotation speed and the corresponding K-L plots for Co-N-C/C4H6CoO4 in O2-saturated 0.5 mol/L H2SO4
|
| 1 | Choi S I, Shao M, Lu N , et al. Synjournal and characterization of Pd@Pt-Ni core-shell octahedra with high activity toward oxygen reduction[J]. ACS Nano, 2016,8(10):126. | | 2 | Wu Z, Lv Y, Xia Y , et al. Ordered mesoporous platinum@graphitic carbon embedded nanophase as a highly active, stable, and methanol-tolerant oxygen reduction electrocatalyst[J]. Journal of the American Chemical Society, 2012,134(4):2236. | | 3 | Bing L, Higgins D C, Xiao Q , et al. The durability of carbon supported Pt nanowire as novel cathode catalyst for a 1.5 kW PEMFC stack[J]. Applied Catalysis B: Environmental, 2015,162:133. | | 4 | Ohyagi S, Sasaki T . Durability of a PEMFC Pt-Co cathode catalyst layer during voltage cycling tests under supersaturated humidity conditions[J]. Electrochimica Acta, 2013,102(102):336. | | 5 | Ma Y, Wang R, Wang H , et al. Evolution of nanoscale amorphous, crystalline and phase-segregated PtNiP nanoparticles and their electrocatalytic effect on methanol oxidation reaction[J]. Physical Che-mistry Chemical Physics, 2014,16(8):3593. | | 6 | Ding W, Li L, Xiong K , et al. Shape fixing via salt recrystallization: A morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction[J]. Journal of the American Chemical Society, 2015,137(16):5414. | | 7 | Proietti E, Jaouen F, Lefèvre M , et al. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells[J]. Nature Communications, 2011,2:416. | | 8 | Jin H, Zhang H, Zhong H , et al. Nitrogen-doped carbon xerogel: A novel carbon-based electrocatalyst for oxygen reduction reaction in proton exchange membrane (PEM) fuel cells[J]. Energy & Environmental Science, 2011,4(9):3389. | | 9 | Deng D, Yu L, Chen X , et al. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2013,52(1):371. | | 10 | Hu Y, Jensen J O, Zhang W , et al. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts[J]. Angewandte Chemie International Edition, 2014,53(14):3675. | | 11 | Elumeeva K, Ren J, Antonietti M , et al. High surface iron/cobalt-containing nitrogen-doped carbon aerogels as non-precious advanced electrocatalysts for oxygen reduction[J]. ChemElectroChem, 2015,2(4):584. | | 12 | Wang Y, Nie Y, Wei Z D . Unification of catalytic oxygen reduction and hydrogen evolution reactions: Highly dispersive Co nanoparticles encapsulated inside Co and nitrogen co-doped carbon[J]. Chemical Communications, 2015,51(43):8942. | | 13 | Yang R, Stevens K, Dahn J R . Investigation of activity of sputtered transition-metal (TM)-C-N (TM=V, Cr, Mn, Co, Ni) catalysts for oxygen reduction reaction[J]. Journal of the Electrochemical Society, 2008,155(1):B79. | | 14 | Zhang H J, Jiang Q Z, Sun L , et al. 3D non-precious metal-based electrocatalysts for the oxygen reduction reaction in acid media[J]. International Journal of Hydrogen Energy, 2010,35(15):8295. | | 15 | Lefèvre M, Proietti E, Jaouen F , et al. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells[J]. Science, 2009,324:71. | | 16 | Peng H, Hou S, Dang D , et al. Ultra-high-performance doped carbon catalyst derived from o-phenylenediamine and the probable roles of Fe and melamine[J]. Applied Catalysis B:Environmental, 2014,158:60. | | 17 | Wu G, Johnston C M, Mack N H , et al. Synjournal-structure-performance correlation for polyaniline-Me-C non-precious metal cathode catalysts for oxygen reduction in fuel cells[J]. Journal of Materials Chemistry, 2011,21:11392. | | 18 | Wu G, More K L, Johnston C M , et al. High-performance electrocatalysts for oxygen reduction derived from polyaniline,iron,and cobalt[J]. Science, 2011,332:443. | | 19 | 殷敬华, 莫志深 . 现代高分子物理学[M]. 北京: 科学出版社, 2001. | | 20 | Lin Senhao, Song Tingwen, Wan Honghe , et al. Ion beam effects in polyaniline films[J].Acta Polymerica Sinica, 1994(1):48(in Chinese). | | 20 | 林森浩, 荣廷文, 万洪和 , 等. 聚苯胺薄膜的离子束效应[J].高分子学报,1994(1):48. | | 21 | Lu Min . Properties and applications of polyaniline[J].Journal of Functional Materials,1998(4):353(in Chinese). | | 21 | 陆珉 . 导电聚苯胺( PAn)的特性及应用[J].功能材料,1998(4):353. | | 22 | Wang G, Jiang K, Xu M , et al. A high activity nitrogen-doped carbon catalyst for oxygen reduction reaction derived from polyaniline-iron coordination polymer[J]. Journal of Power Sources, 2014,266(10):222. | | 23 | Faubert G, C?té R, Guay D , et al. Activation and characterization of Fe-based catalysts for the reduction of oxygen in polymer electrolyte fuel cells[J]. Electrochimica Acta, 1998,43(14-15):1969. | | 24 | Casanovas J, Ricart J M, Rubio J , et al. Origin of the large N 1s binding energy in X-ray photoelectron spectra of calcined carbonaceous materials[J]. Journal of the American Chemical Society, 1996,118(34):8071. | | 25 | Wagner C D, Riggs W W, Davis L E , et al. Handbook of X-ray photoelectron spectroscopy[M]. USA:Perkin-Elmer corporation Physical Electronics Division, 1979: 219. | | 26 | Cochet M, Maser W K, Benito AM , et al. Synjournal of a new polyaniline/nanotube composite: “In-situ” polymerisation and charge transfer through site-selective interaction[J]. Chemical Communications, 2001,16:1450. | | 27 | Tuinstra F, Koenig J L . Raman spectrum of graphite[J]. The Journal of Chemical Physics, 1970,53(3):1126. |
|
|
|
|

 渝公网安备50019002502923号 © Editorial Office of Materials Reports.
渝公网安备50019002502923号 © Editorial Office of Materials Reports.
 2018, Vol. 32
2018, Vol. 32 