| RESEARCH PAPER |
|
 |
|

|
|
|
| Research on Stabilization of Free CaO in Basic Oxygen Furnace Slag with |
| Acidifier at High Temperature SiO2Bearing, , , ,
|
YIN Xiao, ZHANG Chongmin, YANG Ji, LI Boyang, WANG Guocheng
|
|
|
|
|
|
|
Published: 25 January 2018
Online: 2018-01-25
|
|
|
|
|

| FeO | Fe2O3 | CaO | SiO2 | MgO | | 14.68 | 0.70 | 49.38 | 10.56 | 12.00 | | MnO | Al2O3 | P2O5 | Others | | | 5.25 | 2.02 | 2.61 | 2.80 | |
|
|
Chemical compositions (mass fraction/%) of BOFS
|

|
|
Images of experimental materials: (a)BOFS, (b)quartz sand
|

| Content | S1 | S2 | S3 | S4 | S5 | | w(SiO2)/w(CaO) | 0.37 | 0.42 | 0.48 | 0.56 | 0.67 | | w(f-CaO)/% | 2.98 | 2.68 | 2.02 | 1.24 | 0.70 |
|
|
Free CaO content of BOF slag modified at conditions of different w(SiO2)/w(CaO)
|
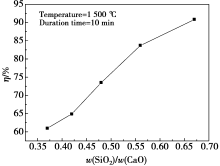
|
|
Relation curve between η and w(SiO2)/w(CaO)
|
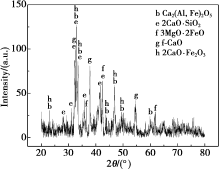
|
|
XRD pattern for BOFS
|

| Mineralogical phase | S1 | S2 | S3 | S4 | S5 | | MgO·Fe2O3 | √ | √ | √ | √ | √ | | CaO·MgO·2SiO2 | √ | — | — | — | — | | 2CaO·MgO·2SiO2 | — | √ | √ | √ | √ | | 2CaO·SiO2 | √ | √ | √ | √ | √ | | 2CaO·Fe2O3 | — | — | — | — | √ | | Ca2(Al,Fe)2O5 | √ | √ | √ | √ | √ | | Fe3O4 | √ | √ | √ | √ | √ | | Fe2O3 | — | — | — | — | √ |
|
|
Mineralogical phases of BOF slag after modification
|

| Sample | CaO | MgO | FeO | MnO | Al2O3 | SiO2 | | BOFS | 81.90 | 5.12 | 8.01 | 4.97 | — | — | | S2 | 79.55 | 5.57 | 6.44 | — | 5.98 | 2.47 |
|
|
EDS results (mass fraction/%) of f-CaO in BOFS and S2
|
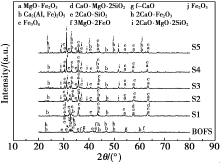
|
|
XRD patterns of BOF slag before and after modification
|

|
|
FESEM images of BOF slag before and after modification
|

| Number | Reaction | Δf/(J·mol-1) | | 1 | SiO2(s)+CaO(s)=
CaO·SiO2(s) | -92 500+2.5T | | 2 | SiO2(s)+2CaO(s)=
2CaO·SiO2(s) | -118 800-11.3T | | 3 | 2SiO2(s)+3CaO(s)=
3CaO·2SiO2(s) | -236 800+9.6T | | 4 | SiO2(s)+3CaO(s)=
3CaO·SiO2(s) | -118 800-6.7T |
|
|
Δf of the main silicates
|

| Content | S1 | S2 | S3 | BOFS | | w(C3S)/w(C2S) | 11.06 | 1.77 | 0.44 | — | | w(CaO)/w(C2S) | 0.29 | 0.19 | 0.08 | 0.98 |
|
|
Theoretical values of w(C3S)/w(C2S) and w(CaO)/w(C2S)
|
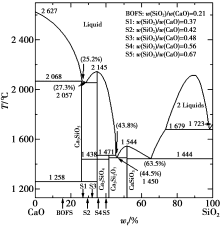
|
|
CaO-SiO2 phase diagram[23]
|

| No. | Source | Form | Size/μm | Characteristics | | 1 | H Suito et al[25] | Undissolved particles | 3—30 | Spongy, grainy | | 2 | F Wachsmuth et al[26] | Precipitate from melt | <4 | Grainy | | 3 | J Waligora et al[5] | Decomposition from C3S | 1—3 | Micro-inclusion | | This work | Before stabilization | Undissolved particles+
Precipitate from melt | 2—20 | Ellipsoid-like | | This work | After stabilization | Precipitate from melt+
Decomposition from C3S | 0.5—2 | Clustered ball-like |
|
|
Classification and characteristics of f-CaO in steel slag
|
| 1 | Li G, Guo M . Current development of slag valorisation in China[J]. Waste & Biomass Valorization, 2014,5(3):317. | | 2 | Yang G, Zhang H . Research on long-term variation tendency of f-CaO content in sodden aging steel slag based on holt-winters[J]. Materials Review B:Research Papers, 2015,29(12):112(in Chinese). | | 3 | 杨刚, 张浩 . 基于三次指数平滑法的水泡陈化钢渣中f-CaO含量长期变化趋势研究[J]. 材料导报:研究篇, 2015,29(12):112. | | 4 | Engstr?m F, Adolfsson D, Yang Q , et al. Crystallization behaviour of some steelmaking slags[J]. Steel Research International, 2010,81(5):362. | | 5 | Xu H J, Fu G Q, Zhu M Y . Experimentation on distensibility of steel slag[J]. Environmental Engineering, 2006,24(6):62(in Chinese). | | 6 | 徐红江, 付贵勤, 朱苗勇 . 钢渣膨胀性的实验[J]. 环境工程, 2006,24(6):62. | | 7 | Waligora J, Bulteel D, Degrugilliers P , et al. Chemical and mineralogical characterizations of LD converter steel slags:A multi-analytical techniques approach[J]. Materials Characterization, 2010,61(1):39. | | 8 | Shi C J . Steel slag-its production, processing, characteristics, and cementitious properties[J]. Journal of Materials in Civil Engineering, 2004,16(3):230. | | 9 | Vaverka J, Sakurai K . Quantitative determination of free lime amount in steelmaking slag by X-ray diffraction[J]. ISIJ Internatio-nal, 2014,54(6):1334. | | 10 | Juckes L M . The volume stability of modern steelmaking slags[J]. Mineral Processing and Extractive Metallurgy, 2003,112(3):177. | | 11 | Geiseler J . Use of steelworks slag in Europe[J]. Waste Management, 1996,16(1-3):59. | | 12 | 10 俞海明, 王强 . 钢渣处理与综合利用[M]. 北京: 冶金工业出版社, 2015: 95. | | 13 | Durinck D, Engstr?m F, Arnout S , et al. Hot stage processing of metallurgical slags[J]. Resources, Conservation and Recycling, 2008,52(10):1121. | | 14 | Wu L, Hao Y D, Zhang K , et al. Exploratory experiment of high efficiency utilization with molten steel slag resource[J]. Environmental Engineering, 2015,33(12):147(in Chinese). | | 15 | 吴龙, 郝以党, 张凯 , 等. 熔融钢渣资源高效化利用探索试验[J]. 环境工程, 2015,33(12):147. | | 16 | Motz H, Geiseler J . Products of steel slags an opportunity to save natural resources[J]. Waste Management, 2001,21(3):285. | | 17 | Peter D, Andreas E, Michael K , et al. Recent development in slag treatment and dust recycling[J]. Steel Research International, 2010,80(10):737. | | 18 | Zhang Y Z, Xing H W, Lei Y B , et al. Study on f-CaO digestion me-chanism of high alkalinity slags with iron ore tailings[J]. Chinese Journal of Environmental Engineering, 2012,6(5):1687(in Chinese). | | 19 | 张玉柱, 邢宏伟, 雷云波 , 等. 高碱度钢渣添加铁尾矿消解f-CaO的机理研究[J]. 环境工程学报, 2012,6(5):1687. | | 20 | 16 Lei Y B, Zhang Y Z, Xing H W, et al. Test to melt and dispel free CaO in converter slag added with powdered coal ash under high temperature[J].Heibei Metallurgy, 2011(1):8(in Chinese). | | 21 | 雷云波, 张玉柱, 邢宏伟 , 等. 转炉渣掺粉煤灰高温熔融消解游离CaO的试验[J].河北冶金, 2011(1):8. | | 22 | Li Jianxin . Effect of modification at high temperature on the composition, structure and property of steel slag[D]. Guangzhou:South China University of Technology, 2010(in Chinese). | | 23 | 李建新 . 高温重构对钢渣组成、结构和性能影响的研究[D]. 广州:华南理工大学, 2010. | | 24 | Li L S . Historical evolution and a vista of trend of converter slag recycling in future[J]. World Iron & Steel, 2011,11(4):62(in Chinese). | | 25 | 李辽沙 . 转炉渣资源化利用的历史沿革及趋势展望[J]. 世界钢铁, 2011,11(4):62. | | 26 | Lu X, Li Y, Ma S , et al. Thermal equilibrium analysis and experiment of molten slag modification by use of its sensible heat[J]. Chinese Journal of Engineering, 2016,38(10):1386(in Chinese). | | 27 | 卢翔, 李宇, 马帅 , 等. 利用显热对熔渣进行直接改质的热平衡分析及试验验证[J]. 工程科学学报, 2016,38(10):1386. | | 28 | Long Y, Lei Y B, Zhang Y Z , et al. Determination of free calcium oxide in steel slag by EDTA complexometric titration[J]. Metallurgical Analysis, 2010,30(7):65(in Chinese). | | 29 | 龙跃, 雷云波, 张玉柱 , 等. EDTA络合滴定法测定钢渣中游离氧化钙[J]. 冶金分析, 2010,30(7):65. | | 30 | 21 王海舟. 炉渣分析[M]. 北京: 科学出版社, 2006: 111. | | 31 | 22 黄希祜. 钢铁冶金原理[M]. 第4版.北京: 冶金工业出版社, 2013: 631. | | 32 | Zaitsev A I, Zemchenko M A, Litvina A D , et al. Thermodynamic calculation of phase equilibria in the CaF2-SiO2-CaO system[J]. Journal of Materials Chemistry, 1993,3(5):541. | | 33 | 4 Yildirim I Z, Prezzi M . Chemical, mineralogical, and morphological properties of steel slag[J]. Advances in Civil Engineering, 2011,2011:1. | | 34 | 5 Inoue R, Suito H . Hydration of crystallized lime in BOF slags[J]. Transactions of the Iron & Steel Institute of Japan, 1995,35(3):272. | | 35 | 6 Wachsmuth F, Geiseler J, Fix W , et al. Contribution to the structure of BOF-slags and its influence on their volume stability[J]. Canadian Metallurgical Quarterly, 1981,20(3):279. |
|
|
|
|

 渝公网安备50019002502923号 © Editorial Office of Materials Reports.
渝公网安备50019002502923号 © Editorial Office of Materials Reports.
 2018, Vol. 32
2018, Vol. 32 