| MATERIALS AND SUSTAINABLE DEVELOPMENT: ADVANCED MATERIALS FOR CLEAN ENERGY UTILIZATION |
|
|

|
|
|
| A Complete Review of Cobalt-based Electrocatalysts Applying to Metal-Air Batteries and Intermediate-Low Temperature Solid Oxide Fuel Cells |
| Wei ZHOU1,2,3,Xixi WANG1,2,3,Yinlong ZHU1,2,3,Jie DAI1,2,3,Yanping ZHU1,2,3,Zongping SHAO1,2,3
|
1 Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing 210009
2 College of Chemical Engineering, Nanjing Tech University, Nanjing 210009
3 State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing 210009 |
|
|
|
|
Abstract The over-exploitation and over-utilization of fossil fuel resources such as petroleum and coal has aggravated energy and environment problem in the 21st century, and urged the development of highly-efficient and cost-effective energy conversion and storage devices to become the research topic of this new era. Among many candidates of energy conversion and storage devices, metal-air batteries and intermediate-low temperature solid oxide fuel cells can efficiently convert chemical energy into electric energy, and enjoy the advantages of low cost, high efficiency and environmental friendliness. Hence, they have provoked intensive and fruitful research endeavors with amazing achievements over the past decade. However, the sluggish kinetics of the oxygen reduction and evolution reactions greatly reduces the energy conversion efficiency, and consequently increases the application cost and severely hinders the commercialization of these two devices. Cobalt-based electrocatalysts, as highly efficient cathode materials with lower cost than noble metals, feature mixed ionic and electronic conductivity which can effectively reduce polarization and contribute to high catalytic activity for oxygen reduction and evolution reactions, and thereby have been holding growing interest recently. For metal-air batteries, cobalt-based electrocatalysts such as simple oxides, spinel oxides, perovskite oxides, and others can significantly improve the discharge capacity and cycle life, and simultaneously, lower the charge voltage and polarization. On the other hand, the catalytic activity and stability need to be further enhanced, and the catalytic mechanisms and active sites deserve further rational exploration and ascertainment. Similarly, cobalt-based electrocatalysts including La1-xSrxCoO3-δ, La1-xSrxCo1-yFeyO3-δ, Ba1-xSrxCoyFe1-yO3-δ and cobalt-based double perovskites show evident efficacy in reducing the cathode polarization resistance and area specific resistance as well as increasing the power density, while nonetheless sustaining a generally higher thermal expansion coefficient and a rather poor stability compared to some other competitors. To further improve the catalytic performance of cobalt-based electrocatalysts for metal-air batteries and intermediate-low tempe-rature solid oxide fuel cells, researchers have developed many useful and productive methods, exemplified by metal elements doping, composite cathode materials preparation, and noble metals decoration. This review provides a brief introduction of the structure and working principle of metal-air batteries and intermediate-low temperature solid oxide fuel cells, and a vivid description upon the latest attempts and achievements for the fabrication, modification and performance of the rich variety of cobalt-based electrocatalysts, mainly including simple oxides, perovskites oxides, spinel oxides and double perovskites.
|
|
Published: 10 February 2018
Online: 2018-02-10
|
|
|
|
|
|
|
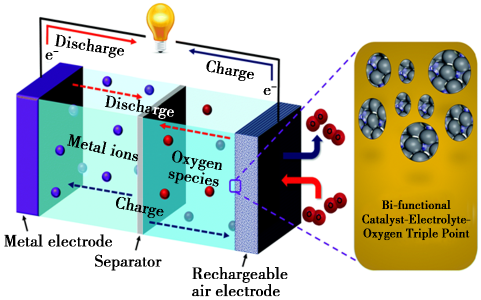
A typical rechargeable metal-air battery based on the bifunctional catalyst and the corresponding working principle<br />
|
|
|
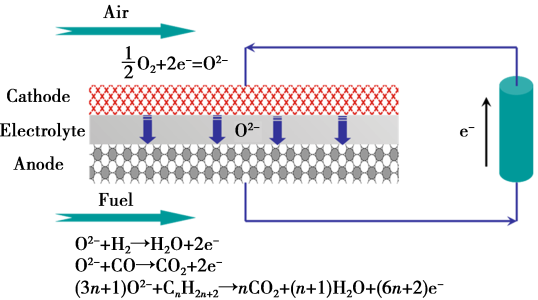
A schematic rendering of the working principle of a solid-oxide fuel cell<br />
|

|
|
Three hypothesized oxygen molecular adsorption modes in the oxygen reduction reaction (ORR): (a) side group type; (b) terminal type; (c) bridge<br />
|
|
|
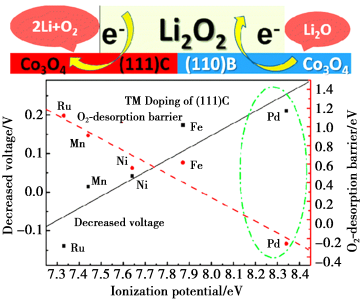
The diagram showing the simulated decomposition reaction mechanism of Li2O2 supported on Co3O4surface<br />
|
|
|
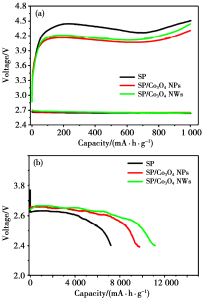
(a) First dischargecharge curves of Li-O2cells with SP,Co3O4 NWs/SP or Co3O4 NPs/SP cathode with a fixed capacity; (b) the first full discharge curves of the Li-O2 cells with SP, Co3O4NWs/SP or Co3O4 NPs/SP cathode<br />
|
| 1 | Tan P, Liu M, Shao Z , et al. Recent advances in perovskite oxides as electrode materials for nonaqueous lithium-oxygen batteries[J]. Advanced Energy Materials, 2017: 1602674. | | 2 | Zhang J, Zhao Z, Xia Z , et al. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions[J]. Nature Nano, 2015,10(5):444. | | 3 | Zheng M B, Qiu D F, Pang H , et al. The progress of studies of li-thium-air batteries[J].Science & Technology Review,2011(14):67(in Chinese). | | 3 | 郑明波, 邱旦峰, 庞欢 , 等. 锂-空气电池研究进展[J].科技导报,2011(14):67. | | 4 | Li Y Y, Wang L, He X M , et al. Research progress of cathode catalyst for lithium-air battery[J]. Chinese Battery Industry, 2014,19(3):163(in Chinese). | | 4 | 李月艳, 王莉, 何向明 , 等. 锂-空气电池正极催化剂研究进展[J]. 电池工业, 2014,19(3):163. | | 5 | Zhou G . Preparation and electrochemical performance of transition metal oxide catalyst for lithium air battery[D]. Changsha:Central South University, 2013(in Chinese). | | 5 | 周耿 . 锂空气电池过渡金属氧化物催化剂的制备及电化学性能研究[D]. 长沙:中南大学, 2013. | | 6 | Cao R, Lee J S, Liu M , et al. Recent progress in non-precious catalysts for metal-air batteries[J]. Advanced Energy Materials, 2012,2(7):816. | | 7 | Zhu M J, Yuan Z S, Sang L , et al. Research progress of metal-air battery[J]. Chinese Journal of Power Sources, 2012,136(12):1953(in Chinese). | | 7 | 朱明骏, 袁振善, 桑林 , 等. 金属/空气电池的研究进展[J]. 电源技术, 2012,136(12):1953. | | 8 | Lee D U, Xu P, Cano Z P , et al. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal-air batteries[J]. Journal of Materials Chemistry A, 2016,4(19):7107. | | 9 | Gao Z, Mogni L V, Miller E C , et al. A perspective on low-tempe-rature solid oxide fuel cells[J]. Energy & Environmental Science, 2016,9(5):1602. | | 10 | Chen Y, Zhou W, Ding D , et al. Advances in cathode materials for solid oxide fuel cells: Complex oxides without alkaline earth metal elements[J]. Advanced Energy Materials, 2015,5(18):1500537. | | 11 | Jiang Z, Xia C, Chen F . Nano-structured composite cathodes for intermediate-temperature solid oxide fuel cells via an infiltration/impregnation technique[J]. Electrochimica Acta, 2010,55(11):3595. | | 12 | Sunarso J, Hashim S S, Zhu N , et al. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review[J]. Progress in Energy and Combustion Science, 2017,61(Supp.C):57. | | 13 | Duan C, Tong J, Shang M , et al. Readily processed protonic cera-mic fuel cells with high performance at low temperatures[J]. Science, 2015,349(6254):1321. | | 14 | Zhang Y, Knibbe R, Sunarso J , et al. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 ℃[J]. Advanced Materials, 2017,29:1700132. | | 15 | Zhou W, Ran R, Shao Z . Progress in understanding and development of Ba0.5Sr0.5Co0.8Fe0.2O3-δ-based cathodes for intermediate-temperature solid-oxide fuel cells: A review[J]. Journal of Power Sources, 2009,192(2):231. | | 16 | Park S, Shao Y, Liu J , et al. Oxygen electrocatalysts for water electrolyzers and reversible fuel cells: Status and perspective[J]. Energy & Environmental Science, 2012,5(11):9331. | | 17 | Zhao H . Preparation of titanium oxides supported catalysts and study on electrochemical performance of air electrode[D]. Hohhot:Inner Mongolian University, 2013(in Chinese). | | 17 | 赵辉 . 钛氧化物载体催化剂的制备及空气电极电化学性能的研究[D]. 呼和浩特:内蒙古大学, 2013. | | 18 | Queiroz A C , Lima F H B. Electrocatalytic activity and stability of Co and Mn-based oxides for the oxygen reduction reaction in alkaline electrolyte[J]. Journal of Electroanalytical Chemistry, 2013,707:142. | | 19 | Storm M M, Overgaard M, Younesi R , et al. Reduced graphene oxide for Li-air batteries: The effect of oxidation time and reduction conditions for graphene oxide[J]. Carbon, 2015,85:233. | | 20 | Lim B, Jiang M, Camargo P H , et al. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction[J]. Science, 2009,324(5932):1302. | | 21 | Su C, Yang T, Zhou W , et al. Pt/C-LiCoO2 composites with ultralow Pt loadings as synergistic bifunctional electrocatalysts for oxygen reduction and evolution reactions[J]. Journal of Materials Che-mistry A, 2016,4(12):4516. | | 22 | Su C, Wang W, Chen Y , et al. SrCo0.9Ti0.1O3-δ as a new electrocatalyst for the oxygen evolution reaction in alkaline electrolyte with stable performance[J]. ACS Applied Materials & Interfaces, 2015,7(32):17663. | | 23 | Zhu Y, Zhou W, Chen Z-G , et al. SrNb0.1Co0.7Fe0.2O3-δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution[J]. Angewandte Chemie International Edition, 2015,54(13):3897. | | 24 | Wang Y, Zhang L M, Hu T J . Progress in oxygen reduction reaction electrocatalysts for metal-air batteries[J].Acta Chimica Sinica,2015(4):316(in Chinese). | | 24 | 王瀛, 张丽敏, 胡天军 . 金属空气电池阴极氧还原催化剂研究进展[J].化学学报,2015(4):316. | | 25 | Yu J, Sunarso J, Zhu Y , et al. Activity and stability of ruddlesden-popper-type Lan+1NinO3n+1 (n=1, 2, 3, and infinity) electrocatalysts for oxygen reduction and evolution reactions in alkaline media[J]. Chemistry-a European Journal, 2016,22(8):2719. | | 26 | Lin Y, Zhou W, Sunarso J , et al. Characterization and evaluation of BaCo0.7Fe0.2Nb0.1O3-δ as a cathode for proton-conducting solid oxide fuel cells[J]. International Journal of Hydrogen Energy, 2012,37(1):484. | | 27 | Zhu Y, Zhou W, Sunarso J , et al. Phosphorus-doped perovskite oxide as highly efficient water oxidation electrocatalyst in alkaline solution[J]. Advanced Functional Materials, 2016,26(32):5862. | | 28 | Chen G, Sunarso J, Zhu Y , et al. Highly active carbon/α-MnO2 hybrid oxygen reduction reaction electrocatalysts[J]. Chemelectrochem, 2016,3(11):1760. | | 29 | Wang D D . Preparation of spinel type metal oxides and their applications in oxygen electrode[D]. Beijing:Beijing University of Chemical Technology, 2013(in Chinese). | | 29 | 王登登 . 尖晶石型金属氧化物的制备及其在氧电极中的应用[D]. 北京:北京化工大学, 2013. | | 30 | Cui Y, Wen Z, Sun S , et al. Mesoporous Co3O4 with different porosities as catalysts for the lithium-oxygen cell[J]. Solid State Ionics, 2012,225:598. | | 31 | Cui Y, Wen Z, Liu Y . A free-standing-type design for cathodes of rechargeable Li-O2 batteries[J]. Energy & Environmental Science, 2011,4(11):4727. | | 32 | Kim K S, Park Y J . Catalytic properties of Co3O4 nanoparticles for rechargeable Li/air batteries[J]. Nanoscale Research Letters, 2012,7(1):47. | | 33 | Débart A, Bao J, Armstrong G , et al. An O2 cathode for rechargeable lithium batteries: The effect of a catalyst[J]. Journal of Power Sources, 2007,174(2):1177. | | 34 | Zhu J, Ren X, Liu J , et al. Unraveling the catalytic mechanism of Co3O4 for the oxygen evolution reaction in a Li-O2 battery[J]. ACS Catalysis, 2015,5(1):73. | | 35 | Liu Q, Jiang Y, Xu J , et al. Hierarchical Co3O4 porous nanowires as an efficient bifunctional cathode catalyst for long life Li-O2batte-ries[J]. Nano Research, 2015,8(2):576. | | 36 | Liang Y, Li Y, Wang H , et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction[J]. Nature Mate-rials, 2011,10(10):780. | | 37 | Black R, Lee J H, Adams B , et al. The role of catalysts and pero-xide oxidation in lithium-oxygen batteries[J]. Angewandte Chemie, 2013,125(1):410. | | 38 | Yoon T H, Park Y J . Carbon nanotube/Co3O4 composite for air electrode of lithium-air battery[J]. Nanoscale Research Letters, 2012,7(1):28. | | 39 | Song M J, Kim I T, Kim Y B , et al. Self-standing, binder-free electrospun Co3O4/carbon nanofiber composites for non-aqueous Li-air batteries[J]. Electrochimica Acta, 2015,182:289. | | 40 | Sun B, Liu H, Munroe P , et al. Nanocomposites of CoO and a mesoporous carbon (CMK-3) as a high performance cathode catalyst for lithium-oxygen batteries[J]. Nano Research, 2012,5(7):460. | | 41 | Liang Y, Wang H, Diao P , et al. Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes[J]. Journal of the American Chemical Society, 2012,134(38):15849. | | 42 | Leng X, Ding X, Hu J , et al. In situ prepared reduced graphene oxide/CoO nanowires mutually-supporting porous structure with enhanced lithium storage performance[J]. Electrochimica Acta, 2016,190:276. | | 43 | Lin S L . Preparation, characterization and study of perovskite-type composite oxides for the photo-electro-catalutic activity[D]. Nanjing:Nanjing University of Science and Technology, 2005(in Chinese). | | 43 | 林生岭 . 钙钛矿复合氧化物的制备、表征及其光电催化活性研究[D]. 南京:南京理工大学, 2005. | | 44 | Kalubarme R S, Kim Y-H, Park C-J. Perovskite composite bifunctional catalyst for rechargeable lithium-oxygen batteries [C]∥Mee-ting Abstracts for 223rd ECS Meeting.Pennington,New Jersey,U.S.:The Electrochemical Society, 2013: 260. | | 45 | Lee J J, Oh M Y, Nahm K S . Effect of ball milling on electrocataly-tic activity of perovskite La0. 6Sr0. 4CoO3-δ applied for lithium air battery[J]. Journal of The Electrochemical Society, 2016,163(2):A244. | | 46 | Li P, Zhang J, Yu Q , et al. One-dimensional porous La0.5Sr0.5-CoO2.91 nanotubes as a highly efficient electrocatalyst for rechargeable lithium-oxygen batteries[J]. Electrochimica Acta, 2015,165:78. | | 47 | Liu G, Chen H, Xia L , et al. Hierarchical mesoporous/macroporous perovskite La0. 5Sr0. 5CoO3-x nanotubes: A bifunctional catalyst with enhanced activity and cycle stability for rechargeable lithium oxygen batteries[J]. ACS Applied Materials & Interfaces, 2015,7(40):22478. | | 48 | Wang Q, Xue Y, Sun S , et al. La0.8Sr0.2Co1-xMnxO3 perovskites as efficient bi-functional cathode catalysts for rechargeable zinc-air batteries[J]. Electrochimica Acta, 2017,254:14. | | 49 | Shimizu Y, Matsuda H, Miura N , et al. Bi-functional oxygen electrode using large surface area perovskite-type oxide catalyst for rechargeable metal-air batteries[J]. Chemistry Letters, 1992,21(6):1033. | | 50 | Shimizu Y, Uemura K, Matsuda H , et al. Bi-functional oxygen electrode using large surface area La1-xCaxCoO3 for rechargeable metal-air battery[J]. Journal of The Electrochemical Society, 1990,137(11):3430. | | 51 | Ohkuma H, Uechi I, Imanishi N , et al. Carbon electrode with perovskite-oxide catalyst for aqueous electrolyte lithium-air secondary batteries[J]. Journal of Power Sources, 2013,223:319. | | 52 | Zhao Y, Xu L, Mai L , et al. Hierarchical mesoporous perovskite La0.5Sr0.5CoO2.91 nanowires with ultrahigh capacity for Li-air batte-ries[J]. Proceedings of the National Academy of Sciences, 2012,109(48):19569. | | 53 | Cheng J, Zhang M, Jiang Y , et al. Perovskite La0.6Sr0.4Co0.2Fe0.8-O3 as an effective electrocatalyst for non-aqueous lithium air batte-ries[J]. Electrochimica Acta, 2016,191:106. | | 54 | Sun N, Liu H, Yu Z , et al. Mn-doped La0.6Sr0.4CoO3 perovskite catalysts with enhanced performances for non-aqueous electrolyte Li-O2 batteries[J]. RSC Advances, 2016,6(16):13522. | | 55 | Wang P X, Shao L, Zhang N Q , et al. Mesoporous CuCo2O4 nano-particles as an efficient cathode catalyst for Li-O2 batteries[J]. Journal of Power Sources, 2016,325:506. | | 56 | Zhang L, Zhang S, Zhang K , et al. Mesoporous NiCo2O4 nanoflakes as electrocatalysts for rechargeable Li-O2 batteries[J]. Chemical Communications, 2013,49(34):3540. | | 57 | Yuan X Z, Qu W, Zhang X , et al. Spinel NixCo2-xO4as a bifunctional air electrode for zinc air batteries[J]. ECS Transactions, 2013,45(29):105. | | 58 | Mohamed S G, Tsai Y-Q, Chen C-J , et al. Ternary spinel MCo2O4 (M=Mn, Fe, Ni, and Zn) porous nanorods as bifunctional cathode materials for lithium-O2 batteries[J]. ACS Applied Materials & Interfaces, 2015,7(22):12038. | | 59 | Li B, Feng J, Qian Y , et al. Mesoporous quasi-single-crystalline NiCo2O4 superlattice nanoribbons with optimizable lithium storage properties[J]. Journal of Materials Chemistry A, 2015,3(19):10336. | | 60 | Lee J S, Nam G, Sun J , et al. Composites of a prussian blue analogue and gelatin-derived nitrogen-doped carbon-supported porous spinel oxides as electrocatalysts for a Zn-Air battery[J]. Advanced Energy Materials, 2016,6(22):1601052. | | 61 | Li N, Yan X, Zhang W , et al. Electrocatalytic activity of spinel-type oxides LiMn2-xCoxO4 with large specific surface areas for metal-air battery[J]. Journal of Power Sources, 1998,74(2):255. | | 62 | Wang L, Zhao X, Lu Y , et al. CoMn2O4 spinel nanoparticles grown on graphene as bifunctional catalyst for lithium-air batteries[J]. Journal of The Electrochemical Society, 2011,158(12):A1379. | | 63 | Liang Y, Wang H, Zhou J , et al. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts[J]. Journal of the American Chemical Society, 2012,134(7):3517. | | 64 | Wang H, Yang Y, Liang Y , et al. Rechargeable Li-O2 batteries with a covalently coupled MnCo2O4-graphene hybrid as an oxygen ca-thode catalyst[J]. Energy & Environmental Science, 2012,5(7):7931. | | 65 | Liu R, Von Malotki C, Arnold L , et al. Triangular trinuclear metal-N4 complexes with high electrocatalytic activity for oxygen reduction[J]. Journal of the American Chemical Society, 2011,133(27):10372. | | 66 | Wu J, Dou S, Shen A , et al. One-step hydrothermal synjournal of NiCo2S4-rGO as an efficient electrocatalyst for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2014,2(48):20990. | | 67 | Chen Z, Choi J-Y, Wang H , et al. Highly durable and active non-precious air cathode catalyst for zinc air battery[J]. Journal of Power Sources, 2011,196(7):3673. | | 68 | Liu Y, Jiang H, Zhu Y , et al. Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions[J]. Journal of Materials Chemistry A, 2016,4(5):1694. | | 69 | Ahn C-H, Okada T, Ishida M , et al. Electrochemical characteristic of based on carbon mixed with organic metal complex (Co (mqph)) in alkaline media Li-air battery[J]. Journal of Power Sources, 2016,307:474. | | 70 | Sun C, Hui R, Roller J . Cathode materials for solid oxide fuel cells: A review[J]. Journal of Solid State Electrochemistry, 2010,14(7):1125. | | 71 | Peng R, Wu T, Liu W , et al. Cathode processes and materials for solid oxide fuel cells with proton conductors as electrolytes[J]. Journal of Materials Chemistry, 2010,20(30):6218. | | 72 | Shao Z P . Cathode materials for solid oxide fuel cells towards opera-ting at intermediate-to-low temperature range[J]. Progress in Chemistry, 2011,23(2-3):418(in Chinese). | | 72 | 邵宗平 . 中低温固体氧化物燃料电池阴极材料[J]. 化学进展, 2011,23(2-3):418. | | 73 | Zhou W, Shao Z P, Ran R , et al. Functional nano-composite oxides synthesized by environmental-friendly auto-combustion within a micro-bioreactor[J]. Materials Research Bulletin, 2008,43(8-9):2248. | | 74 | Zhou W, Ran R, Shao Z , et al. Barium-and strontium-enriched (Ba0.5Sr0.5)1+xCo0.8Fe0.2O3-δ oxides as high-performance cathodes for intermediate-temperature solid-oxide fuel cells[J]. Acta Materialia, 2008,56(12):2687. | | 75 | Zhou W, Ran R, Shao Z , et al. Evaluation of A-site cation-deficient (Ba0.5Sr0.5)(1-κ)Co0.8Fe0.2O3-δ(κ> 0) perovskite as a solid-oxide fuel cell cathode[J]. Journal of Power Sources, 2008,182(1):24. | | 76 | Zhou W, Zhao M, Liang F , et al. High activity and durability of novel perovskite electrocatalysts for water oxidation[J]. Materials Horizons, 2015,2(5):495. | | 77 | Ge L, Yang Y, Wang L , et al. High activity electrocatalysts from metal-organic framework-carbon nanotube templates for the oxygen reduction reaction[J]. Carbon, 2015,82:417. | | 78 | Shao Z, Zhou W, Zhu Z . Advanced synjournal of materials for intermediate-temperature solid oxide fuel cells[J]. Progress in Materials Science, 2012,57(4):804. | | 79 | Mizusaki J, Tabuchi J, Matsuura T , et al. Electrical conductivity and Seebeck coefficient of nonstoichiometric La1-xSrxCoO3-δ[J]. Journal of the Electrochemical Society, 1989,136(7):2082. | | 80 | Mineshige A, Kobune M, Fujii S , et al. Metal-insulator transition and crystal structure of La1-xSrxCoO3 as functions of Sr-content, temperature, and oxygen partial pressure[J]. Journal of Solid State Chemistry, 1999,142(2):374. | | 81 | Ralph J M, Rossignol C, Kumar R . Cathode materials for reduced-temperature SOFCs[J]. Journal of The Electrochemical Society, 2003,150(11):A1518. | | 82 | Inagaki T, Miura K, Yoshida H , et al. High-performance electrodes for reduced temperature solid oxide fuel cells with doped lanthanum gallate electrolyte: II. La(Sr)CoO3 cathode[J]. Journal of Power Sources, 2000,86(1):347. | | 83 | Evans A, Martynczuk J, Stender D , et al. Low-temperature micro-solid oxide fuel cells with partially amorphous La0.6Sr0.4CoO3-δ cathodes[J]. Advanced Energy Materials, 2015,5(1):1400747. | | 84 | Acu?a L M, Mu?oz F F, Fuentes R O . Correlation between structural, chemical, and electrochemical properties of La0.6Sr0.4CoO3-d nanopowders for application in intermediate temperature solid oxide fuel cells[J]. The Journal of Physical Chemistry C, 2016,120(36):20387. | | 85 | Zeng R, Huang Y . Enhancing surface activity of La0.6Sr0.4CoO3-δ cathode by a simple infiltration process[J]. International Journal of Hydrogen Energy, 2017,42(10):7220. | | 86 | Hwang J, Lee H, Yoon K J , et al. Study on the electrode reaction mechanism of pulsed-laser deposited thin-film La1-xSrxCoO3-δ (x=0.2. 0.4) cathodes[J]. Journal of the Electrochemical Society, 2012,159(10):F639. | | 87 | Park J-S, Bae J, Hong S , et al. Superior La1-xSrxCoO3-δ ceramic electrode fabrication by MOCSD for low-temperature SOFC application[J]. Surface and Coatings Technology, 2017,311:157. | | 88 | Gwon O, Yoo S, Shin J , et al. Optimization of La1-xSrxCoO3-δ perovskite cathodes for intermediate temperature solid oxide fuel cells through the analysis of crystal structure and electrical properties[J]. International Journal of Hydrogen Energy, 2014,39(35):20806. | | 89 | Tao Y, Shao J, Wang W G , et al. Optimisation and evaluation of La0.6Sr0.4CoO3-δ cathode for intermediate temperature solid oxide fuel cells[J]. Fuel Cells, 2009,9(5):679. | | 90 | Kim Y T, Shikazono N . Investigation of La0.6Sr0.4CoO3-δ-Gd0.1Ce0.9O2-δ composite cathodes with different volume ratios by three dimensional reconstruction[J]. Solid State Ionics, 2017,309:77. | | 91 | Zhao F, Peng R, Xia C . A La0.6Sr0.4CoO3-δ-based electrode with high durability for intermediate temperature solid oxide fuel cells[J]. Materials Research Bulletin, 2008,43(2):370. | | 92 | Matsuda M, Ihara K, Miyake M . Influences of Ga doping on lattice parameter, microstructure, thermal expansion coefficient and electrical conductivity of La0.6Sr0.4CoO3-y[J]. Solid State Ionics, 2004,172(1):57. | | 93 | Lee K T, Manthiram A . Comparison of Ln0.6Sr0.4CoO3-δ (Ln=La, Pr, Nd, Sm, and Gd) as cathode materials for intermediate tempe-rature solid oxide fuel cells[J]. Journal of The Electrochemical Society, 2006,153(4):A794. | | 94 | Teraoka Y, Zhang H M, Okamoto K , et al. Mixed ionic-electronic conductivity of La1-xSrxCo1-yFeyO3-δ perovskite-type oxides[J]. Materials Research Bulletin, 1988,23(1):51. | | 95 | Stevenson J W, Armstrong T R, Carneim R D , et al. Electrochemical properties of mixed conducting perovskites La1-xMxCo1-y-FeyO3-δ (M=Sr, Ba, Ca)[J]. Journal of The Electrochemical Society, 1996,143(9):2722. | | 96 | Steele B C H. Survey of materials selection for ceramic fuel cells II. Cathodes and anodes[J].Solid State Ionics,1996,86-88(Part 2):1223. | | 97 | Sahibzada M, Benson S J, Rudkin R A , et al. Pd-promoted La0.6-Sr0.4Co0.2Fe0.8O3 cathodes[J].Solid State Ionics, 1998, 113- 115:285. | | 98 | Han G D, Neoh K C, Bae K , et al. Fabrication of lanthanum strontium cobalt ferrite (LSCF) cathodes for high performance solid oxide fuel cells using a low price commercial inkjet printer[J]. Journal of Power Sources, 2016,306:503. | | 99 | Mani R, Gautam R, Banerjee S , et al. A study on La0.6Sr0.4Co0.3Fe0.8O3 (LSCF) cathode material prepared by gel combustion method for IT-SOFCs: Spectroscopic, electrochemical and microstructural analysis[J]. Asian Journal of Research in Chemistry, 2015,8(6):389. | | 100 | Wang H, Yakal-Kremski K J, Yeh T, et al. Mechanisms of performance degradation of (La,Sr)(Co,Fe)O3-δ solid oxide fuel cell cathodes[J]. Journal of The Electrochemical Society, 2016,163(6):F581. | | 101 | Liu Y, Cao Y, Yang S , et al. Effects of oxygen partial pressure on the performance stability of impregnated La0.6Sr0.4Co0.2Fe0.8O3-δ-Sm0.2Ce0.8O2 cathodes of solid oxide fuel cells[J]. Fuel Processing Technology, 2015,135:203. | | 102 | Kammer K . Studies of Fe-Co based perovskite cathodes with diffe-rent A-site cations[J]. Solid State Ionics, 2006,177(11):1047. | | 103 | Chen K, Li N, Ai N , et al. Polarization-induced interface and Sr segregation of in situ assembled La0.6Sr0.4Co0.2Fe0.8O3-δ electrodes on Y2O3-ZrO2 electrolyte of solid oxide fuel cells[J]. ACS Applied Materials & Interfaces, 2016,8(46):31729. | | 104 | Wang W G, Mogensen M . High-performance lanthanum-ferrite-based cathode for SOFC[J]. Solid State Ionics, 2005,176(5):457. | | 105 | Dusastre V, Kilner J A . Optimisation of composite cathodes for intermediate temperature SOFC applications[J]. Solid State Ionics, 1999,126(1):163. | | 106 | Hua C-H, Chou C-C . Preparation of nanoscale composite LSCF/GDCS cathode materials by microwave sintering for intermediate-temperature SOFC applications[J]. Ceramics International, 2015,41(Supp.1):S708. | | 107 | Xi X, Kondo A, Kozawa T , et al. LSCF-GDC composite particles for solid oxide fuel cells cathodes prepared by facile mechanical method[J]. Advanced Powder Technology, 2016,27(2):646. | | 108 | Leng Y J, Chan S H, Jiang S P , et al. Low-temperature SOFC with thin film GDC electrolyte prepared in situ by solid-state reaction[J]. Solid State Ionics, 2004,170(1):9. | | 109 | Jia C, Chen M, Han M . Performance and electrochemical analysis of solid oxide fuel cells based on LSCF-YSZ nano-electrode[J]. International Journal of Applied Ceramic Technology, 2017,14(5):1006. | | 110 | Gao C, Liu Y, Xi K , et al. Improve the catalytic property of La0.6-Sr0.4Co0.2Fe0.8O3/Ce0.9Gd0.1O2 (LSCF/CGO) cathodes with CuO nanoparticles infiltration[J]. Electrochimica Acta, 2017,246:148. | | 111 | Burnwal S K, Bharadwaj S, Kistaiah P . Review on MIEC cathode materials for solid oxide fuel cells[J]. Journal of Molecular and Engineering Materials, 2016,04(02):1630001. | | 112 | Shao Z, Haile S M . A high-performance cathode for the next gene-ration of solid-oxide fuel cells[J]. Nature, 2004,431:170. | | 113 | Wei B, Lü Z, Huang X , et al. Crystal structure, thermal expansion and electrical conductivity of perovskite oxides BaxSr1-xCo0.8-Fe0.2O3-δ(0.3≤x≤0.7)[J]. Journal of the European Ceramic Society, 2006,26(13):2827. | | 114 | Chen Z, Ran R, Zhou W , et al. Assessment of Ba0.5Sr0.5Co1-y-FeyO3-δ (y=0.0-1.0) for prospective application as cathode for IT-SOFCs or oxygen permeating membrane[J]. Electrochimica Acta, 2007,52(25):7343. | | 115 | Fisher C a J, Yoshiya M, Iwamoto Y , et al. Oxide ion diffusion in perovskite-structured Ba1-xSrxCo1-yFeyO2.5: A molecular dyna-mics study[J]. Solid State Ionics, 2007,177(39):3425. | | 116 | Li S, Lü Z, Wei B , et al. A study of (Ba0.5Sr0.5)1-xSmxCo0.8Fe0.2-O3-δ as a cathode material for IT-SOFCs[J]. Journal of Alloys and Compounds, 2006,426(1):408. | | 117 | Li S, Lü Z, Ai N , et al. Electrochemical performance of (Ba0.5-Sr0.5)0.9Sm0.1Co0.8Fe0.2O3-δ as an intermediate temperature solid oxide fuel cell cathode[J]. Journal of Power Sources, 2007,165(1):97. | | 118 | Li S, Lü Z, Huang X , et al. Electrical and thermal properties of (Ba0.5-Sr0.5)1-xSmxCo0.8Fe0.2O3-δ perovskite oxides[J]. Solid State Ionics, 2007,178(5):417. | | 119 | Ding X, Kong X, Jiang J , et al. Characterization and electrochemical performance of (Ba0.6Sr0.4)1-xLaxCo0.6Fe0.4O3-δ (x=0, 0.1) cathode for intermediate temperature solid oxide fuel cells[J]. Materials Research Bulletin, 2010,45(9):1271. | | 120 | Meng X, Meng B, Tan X , et al. Synjournal and properties of Ba0.5-Sr0.5(Co0.6Zr0.2)Fe0.2O3-δperovskite cathode material for intermediate temperature solid-oxide fuel cells[J]. Materials Research Bulletin, 2009,44(6):1293. | | 121 | He Y, Fan L, Afzal M , et al. Cobalt oxides coated commercial Ba0.5Sr0.5Co0.8Fe0.2O3-δ as high performance cathode for low-temperature SOFCs[J]. Electrochimica Acta, 2016,191:223. | | 122 | Popov M P, Starkov I A, Bychkov S F , et al. Improvement of Ba0.5Sr0.5Co0.8Fe0.2O3-δ functional properties by partial substitution of cobalt with tungsten[J]. Journal of Membrane Science, 2014,469(Supp.C):88. | | 123 | Popov M P, Bychkov S F, Nemudry A P . Modification of mixed conducting Ba0.5Sr0.5Co0.8Fe0.2O3-δ by partial substitution of cobalt with tungsten[J]. Russian Journal of Electrochemistry, 2016,52(7):648. | | 124 | Li J, Yang C, Liu M . High performance intermediate temperature solid oxide fuel cells with Ba0.5Sr0.5Co0.8Fe0.1Nb0.1O3-δ as cathode[J]. Ceramics International, 2016,42(16):19397. | | 125 | Zhou W, Shao Z, Ran R , et al. Ba0.5Sr0.5Co0.8Fe0.2O3-δ+LaCoO3 composite cathode for Sm0.2Ce0.8O1.9-electrolyte based intermediate-temperature solid-oxide fuel cells[J]. Journal of Power Sources, 2007,168(2):330. | | 126 | Lee S O, Lee D, Jung I , et al. Ceria interlayer-free Ba0.5Sr0.5Co0.8-Fe0.2O3-δ-Sc0.1Zr0.9O1.95composite cathode on zirconia based electrolyte for intermediate temperature solid oxide fuel cells[J]. International Journal of Hydrogen Energy, 2013,38(22):9320. | | 127 | Giuliano A, Carpanese M P, Panizza M , et al. Characterisation of La0.6Sr0.4Co0.2Fe0.8O3-δ-Ba0.5Sr0.5Co0.8Fe0.2O3-δ composite as cathode for solid oxide fuel cells[J]. Electrochimica Acta, 2017,240:258. | | 128 | Wei B, Lü Z, Li S , et al. Thermal and electrical properties of new cathode material Ba0.5Sr0.5Co0.8Fe0.2O3-δ for solid oxide fuel cells[J]. Electrochemical and Solid-State Letters, 2005,8(8):A428. | | 129 | Mosia?ek M., K?dra A., Krzan M ., et al. Ba0.5Sr0.5Co0.8Fe0.2-O3-δ-La0.6Sr0.4Co0.8Fe0.2O3-δ composite cathode for solid oxide fuel cell[J]. Archives of Metallurgy and Materials, 2016,61(3):1483. | | 130 | Wei F, Jiang J, Yu G , et al. BSCF based nanocomposite cathodes fabricated by ion-impregnating method for solid oxide fuel cells[J]. International Journal of Electrochemical Science, 2015,10:7159. | | 131 | Kao W-X, Lee M-C, Lin T-N , et al. Fabrication and characterization of a Ba0.5Sr0.5Co0.8Fe0.2O3-δ-gadolinia-doped ceria cathode for an anode-supported solid-oxide fuel cell[J]. Journal of Power Sources, 2010,195(8):2220. | | 132 | Meng G, Jiang C, Ma J , et al. Comparative study on the perfor-mance of a SDC-based SOFC fueled by ammonia and hydrogen[J]. Journal of Power Sources, 2007,173(1):189. | | 133 | Li K, Wang X, Jia L , et al. High performance Ni-Fe alloy supported SOFCs fabricated by low cost tape casting-screen printing-cofiring process[J]. International Journal of Hydrogen Energy, 2014,39(34):19747. | | 134 | Ai N, Jiang S P, Lü Z , et al. Nanostructured (Ba,Sr)(Co,Fe)-O3-δ impregnated (La,Sr)MnO3 cathode for intermediate-temperature solid oxide fuel cells[J]. Journal of the Electrochemical Society, 2010,157(7):B1033. | | 135 | Zhou W, Ran R, Shao Z , et al. Electrochemical performance of silver-modified Ba0.5Sr0.5Co0.8Fe0.2O3-δ cathodes prepared via electroless deposition[J]. Electrochimica Acta, 2008,53(13):4370. | | 136 | Lin Y, Ran R, Shao Z . Silver-modified Ba0.5Sr0.5Co0.8Fe0.2O3-δ as cathodes for a proton conducting solid-oxide fuel cell[J]. International Journal of Hydrogen Energy, 2010,35(15):8281. | | 137 | Chen Y, Wang F, Chen D , et al. Role of silver current collector on the operational stability of selected cobalt-containing oxide electrodes for oxygen reduction reaction[J]. Journal of Power Sources, 2012,210(Supplement C):146. | | 138 | Mosia?ek M, Dudek M, Michna A , et al. Composite cathode materials Ag-Ba0.5Sr0.5Co0.8Fe0.2O3 for solid oxide fuel cells[J]. Journal of Solid State Electrochemistry, 2014,18(11):3011. | | 139 | Kim J-H, Manthiram A . LnBaCo2O5+δ oxides as cathodes for intermediate-temperature solid oxide fuel cells[J]. Journal of the Electrochemical Society, 2008,155(4):B385. | | 140 | Zhang K, Ge L, Ran R , et al. Synjournal, characterization and eva-luation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs[J]. Acta Materialia, 2008,56(17):4876. | | 141 | Wang W, Peh T S, Chan S H , et al. Synjournal and characterization of LnBaCo2O5+δ layered perovskites as cathodes for intermediate-temperature solid oxide fuel cells[J]. ECS Transactions, 2009,25(2):2277. | | 142 | Kim J H, Cassidy M , Irvine J T S, et al. Advanced electrochemical properties of LnBa0.5Sr0.5Co2O5+δ (Ln=Pr, Sm, and Gd) as cathode materials for IT-SOFC[J]. Journal of the Electrochemical Society, 2009,156(6):B682. | | 143 | Che X, Shen Y, Li H , et al. Assessment of LnBaCo1.6Ni0.4O5+δ (Ln=Pr, Nd, and Sm) double-perovskites as cathodes for intermediate-temperature solid-oxide fuel cells[J]. Journal of Power Sources, 2013,222:288. | | 144 | Chen D, Ran R, Shao Z . Assessment of PrBaCo2O5+δ+Sm0.2Ce0.8-O1.9 composites prepared by physical mixing as electrodes of solid oxide fuel cells[J]. Journal of Power Sources, 2010,195(21):7187. | | 145 | Kim J H, Cassidy M , Irvine J T S, et al. Electrochemical investigation of composite cathodes with SmBa0.5Sr0.5Co2O5+δ cathodes for intermediate temperature-operating solid oxide fuel cell[J]. Chemistry of Materials, 2010,22(3):883. | | 146 | Choi S, Yoo S, Kim J , et al. Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa(0.5)Sr(0.5)-Co(2-x)Fe(x)O(5+δ)[J]. Scientific Reports, 2013,3:2426. | | 147 | Munoz-Gil D, Perez-Coll D, Urones-Garrote E , et al. Influence of the synjournal conditions on the crystal structure and properties of GdBaCo2-xFexO5+δ oxides as air-electrodes for intermediate temperature solid oxide fuel cells[J]. Journal of Materials Chemistry A, 2017,5(24):12550. | | 148 | Anjum U, Khatoon N, Sardar M , et al. Nanoparticle synjournal and oxygen anion diffusion in double perovskite GdBaCo2-xFexO5+δ electrodes for SOFC[J]. ECS Transactions, 2016,72(7):111. | | 149 | Jin F, Shen Y, Wang R , et al. Double-perovskite PrBaCo2/3Fe2/3-Cu2/3O5+δ as cathode material for intermediate-temperature solid-oxide fuel cells[J]. Journal of Power Sources, 2013,234:244. | | 150 | Jo S H, Muralidharan P, Kim D K . Enhancement of electrochemical performance and thermal compatibility of GdBaCo2/3Fe2/3Cu2/3-O5+δ cathode on Ce1.9Gd0.1O1.95 electrolyte for IT-SOFCs[J]. Electrochemistry Communications, 2009,11(11):2085. | | 151 | Lee T-H, Park K-Y, Kim N-I , et al. Robust NdBa0.5Sr0.5Co1.5-Fe0.5O5+δ cathode material and its degradation prevention operating logic for intermediate temperature-solid oxide fuel cells[J]. Journal of Power Sources, 2016,331:495. |
|
|
|
|

 渝公网安备50019002502923号 © Editorial Office of Materials Reports.
渝公网安备50019002502923号 © Editorial Office of Materials Reports.
 2018, Vol. 32
2018, Vol. 32 