| MATERIALS AND SUSTAINABLE DEVELOPMENT: ADVANCED MATERIALS FOR CLEAN ENERGY UTILIZATION |
|
 |
|

|
|
|
| Construction of Nano-Au@PANI Yolk-shell Hollow Structure Electrode Material and Its Electrochemical Performance |
Yongtao TAN1,2( ),Lingbin KONG1,2,Long KANG1,2,Fen RAN1,2 ),Lingbin KONG1,2,Long KANG1,2,Fen RAN1,2
|
1 College of Materials Science and Engineering, Lanzhou University of Technology, Lanzhou 730050
2 State Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metals, Lanzhou University of Technology, Lanzhou 730050 |
|
|
|
|
Abstract The nano-Au@PANI was prepared via a two-step method of oxidative polymerization by controlling the diffusion of oxidizing agents. The morphology of nano-Au@PANI was characterized by TEM, and the performances of supercapacitor were measured by electrochemical work-station (CHI660E). Furthermore, the relationship of reaction time and the performances of supercapacitor were also studied. The results showed that the nano-Au@PANI composites possessed yolk-shell structure, and with extension of reaction time the specific capacitance first increased and then decreased. When the reaction time was 12 h, the shell thickness of PANI was about 21 nm, the specific capacitance was up to 79 F·g -1.
|
|
Published: 10 January 2018
Online: 2018-01-10
|
|
|
|
|
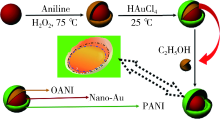
|
|
Symmetric mechanism of Nano-Au@PANI yolk-shell structure
|
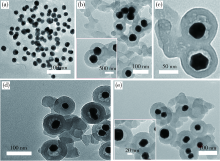
|
|
TEM photos of (a)Nano-Au, (b)Nano-Au@PANI-6,(c)Nano-Au@PANI-12, (d)Nano-Au@PANI-36 and (e)Nano-Au@PANI-48
|
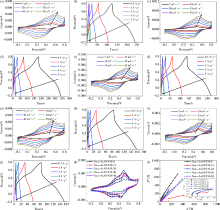
|
|
Electrochemical performance of (a, b) Nano-Au@PANI-6, (c, d) Nano-Au@PANI-12, (e, f) Nano-Au@PANI-24,(g, h) Nano-Au@PANI-36, (i, j) Nano-Au@PANI-48; (k) CV curves of Nano-Au@PANI at different time (scan rate: 5 mV·s-1) and (h)Nyquist plots
|
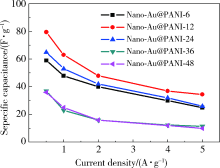
|
|
Specific capacitances of composites at different current density
|
| [1] | Simon P, Gogotsi Y . Materials for electrochemical capacitor[J]. Nature Materials, 2008,7(11):845. | | [2] | Burke A . Ultracapacitors: Why, how, and where is the technology[J]. Journal of Power Sources, 2000,91(1):37. | | [3] | Zhu Y, Murali S, Stoller M D , et al. Carbon-based supercapacitors produced by activation of graphene[J]. Science, 2011,332(6037):1537. | | [4] | Zhang L L, Zhao X S . Carbon-based materials as supercapacitor electrodes[J]. Chemical Society Reviews, 2009,38(9):2520. | | [5] | Zhi M, Xiang C, Li J , et al. Nanostructured carbon-metal oxide composite electrodes for supercapacitors: A review[J]. Nanoscale, 2013,5(1):72. | | [6] | Wang J G, Kang F, Wei B . Engineering of MnO2-based nanocomposites for high-performance supercapacitors[J]. Progress in Materials Science, 2015,74:51. | | [7] | Snook G A, Kao P, Best A S . Conducting-polymer-based supercapacitor devices and electrodes[J]. Journal of Power Sources, 2011,196(1):1. | | [8] | Kimizuka O, Tanaike O, Yamashita J , et al. Electrochemical doping of pure single-walled carbon nanotubes used as supercapacitor electrodes[J]. Carbon, 2008,46(14):1999. | | [9] | Xia K, Gao Q, Jiang J , et al. Hierarchical porous carbons with controlled micropores and mesopores for supercapacitor electrode materials[J]. Carbon, 2008,46(13):1718. | | [10] | EnterriaM, Pereira M F R, Martins J I , et al. Hydrothermal functionalization of ordered mesoporous carbons:The effect of boron on supercapacitor performance[J]. Carbon, 2015,95:72. | | [11] | LvW, Li Z, Deng Y , et al. Graphene-based materials for electrochemical energy storage devices: Opportunities and challenges[J]. Energy Storage Materials, 2016,2:107. | | [12] | GhoshA, Lee Y H . Carbon-based electrochemical capacitors[J]. ChemSusChem, 2012,5(3):480. | | [13] | ShanM L, Liu Y J, Li X , et al. KOH-activated carbons used as electrode materials for supercapacitor Material Review A: Review Papers, 2016,30(5):11(in Chinese). | | [13] | 单明礼, 刘玉静, 李霞 , 等. 氢氧化钾改性碳材料及其在超级电容器中的应用[J]. 材料导报:综述篇, 2016,30(5):11. | | [14] | FengC C, Wu A M, Huang H . Recent progress of N-doped porous carbon materials with applications to supercapacitor electrode Material Review A: Review Papers, 2016,30(1):143(in Chinese). | | [14] | 冯晨辰, 吴爱民, 黄昊 . 超级电容器电极用氮-掺杂多孔碳材料的研究进展[J]. 材料导报:综述篇, 2016,30(1):143. | | [15] | HuangM, Li F, Dong F , et al. MnO2-based nanostructures for high-performance supercapacitors[J]. Journal of Materials Chemistry A, 2015,3(43):21380. | | [16] | SuX H, Yu L, Cheng G . Hydrothermally synthesized manganese dioxide film as supercapacitor electrode Material Review B: Research Papers, 2015,29(5):18(in Chinese). | | [16] | 苏小辉, 余林, 程高 . 水热合成法制备超级电容器用二氧化锰薄膜电极[J]. 材料导报:研究篇, 2015,29(5):18. | | [17] | LangJ W, Kong L B, Wu W J , et al. Facile approach to prepare loose-packed NiO nano-flakes materials for supercapacitors[J]. Chemical Communications, 2008,35(35):4213. | | [18] | KongL B, Zhang J, An J J , et al. MWNTs/PANI composite materials prepared by in-situ chemical oxidative polymerization for supercapacitor electrode[J]. Journal of Materials Science, 2008,43(10):3664. | | [19] | ZhangJ, Kong L B, Li H , et al. Synjournal of polypyrrole film by pulse galvanostatic method and its application as supercapacitor electrode materials[J]. Journal of Materials Science, 2010,45(7):1947. | | [20] | BerzinaT, Pucci A, Ruggeri G , et al. Gold nanoparticles-polyaniline composite material:Synjournal, structure and electrical properties[J]. Synthetic Metals, 2011,161(13-14):1408. | | [21] | HasanM, Ansari M O, Cho M H , et al. Electrical conductivity, optical property and ammonia sensing studies on HCl doped Au@polyaniline nanocomposites[J]. Electronic Materials Letters, 2015,11(1):1. | | [22] | ZhangL, Peng H, Kilmartin P A , et al. Self-assembled hollow polyaniline/Au nanospheres obtained by a one-step synjournal[J]. Macromolecular Rapid Communications, 2008,29(7):598. | | [23] | WangX, Shen Y, Xie A , et al. Assembly of dandelion-like Au/PANI nanocomposites and their application as SERS nanosensors[J]. Biosensors & Bioelectronics, 2011,26(6):3063. | | [24] | SunH, Shen X, Yao L , et al. Measuring the unusually slow ionic diffusion in polyaniline via study of yolk-shell nanostructures[J]. Journal of the American Chemical Society, 2012,134(27):11243. | | [25] | LangX, Zhang L, Fujita T , et al. Three-dimensional bicontinuous nanoporous Au/polyaniline hybrid films for high-performance electrochemical supercapacitors[J]. Journal of Power Sources, 2012,197:325. | | [26] | NobregaM M, Martins V L, Torresi R M , et al. One-step synjournal, characterization, and properties of emeraldine salt nanofibers containing gold[J]. The Journal of Physical Chemistry C, 2014,118(8):4267. | | [27] | HeJ, Liu Y, Babu T , et al. Self-assembly of inorganic nanoparticle vesicles and tubules driven by tethered linear block copolymers[J]. Journal of the American Chemical Society, 2012,134(28):11342. | | [28] | FrensG, , Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions[J]. Nature Physical Science, 1973,241(105):20. | | [29] | HuC C, Lin J Y . Effects of the loading and polymerization temperature on the capacitive performance of polyaniline in NaNO3[J]. Electrochimica Acta, 2002,47(25):4055. | | [30] | WangY G, Li H Q, Xia Y Y . Ordered whiskerlike polyaniline grown on the surface of mesoporous carbon and its electrochemical capacitance performance[J]. Advanced Materials, 2006,18(19):2619. |
|
|
|
|

 渝公网安备50019002502923号 © Editorial Office of Materials Reports.
渝公网安备50019002502923号 © Editorial Office of Materials Reports.
 2018, Vol. 32
2018, Vol. 32 
